[ad_1]
A oxide It is a chemical compound that arises from the combinations of a metallic or non-metallic element with oxygen. For instance: calcium oxide (combination of calcium and oxygen), sulfur dioxide (combination of sulfur and oxygen).
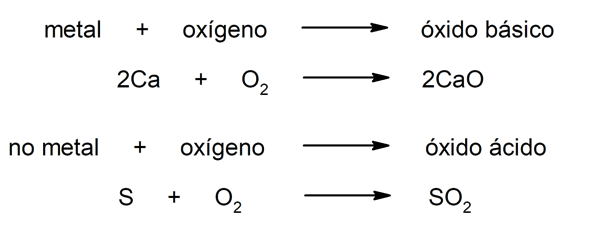
Oxides are usually formed when chemical elements combine with air or water, which have a large presence of oxygen, which causes wear on the elements, especially when it comes to metals. To remedy this, antioxidant substances are often used.
Within the oxides, a classification according to the element with which oxygen is combined:
- Basic oxides. They are chemical compounds that are obtained by combining a metal element with oxygen.
- Acid oxides. They are chemical compounds that are obtained by combining a non-metal element with oxygen.
- Amphoteric oxides. They are formed by an amphoteric element and oxygen, so these oxides act as acids or bases depending on the reaction in which they are involved.
Nomenclature of oxides
To name these types of substances, there are three possible ways to do it:
- The traditional (or stoichiometric) nomenclature
Add a series of prefixes and suffixes to the name of the element (metallic or non-metallic) depending on the amount of oxidation numbers it has.
Also, if it is a basic oxide it is written ‘oxide of (name of the metal with its respective prefixes and suffixes)’ and if it is an acid oxide it is usually also written ‘anhydride (name of the metal with its respective prefixes and suffixes)’.
When the element has only one oxidation number:
- ‘oxide (and the element with the built-in suffix -ico) ‘. For instance: potassium oxide (K2OR).
When the element has two oxidation numbers:
- For the highest oxidation number: ‘oxide (and the element with the built-in suffix -ico)’. For instance: ferric oxide (Fe2OR3)
- For the lowest oxidation number: ‘oxide (and the element with the built-in suffix -oso)’. For instance: ferrous oxide (FeO).
When the element has three oxidation numbers:
- For the lowest oxidation number: ‘oxide (and the element with the prefix’ hypo ‘and the suffix -oso)’. For instance: hyposulfurous anhydride (SO)
- For the intermediate oxidation number: ‘oxide (and the element with the suffix -oso) For example: sulfur dioxide (SO2).
- For the highest oxidation number: ‘oxide (and the element with the built-in suffix -ico). For instance: sulfuric anhydride (SO3). In this case we use the word ‘anhydride’ because sulfur is a non-metal, because we are talking about acid oxides.
When the element has four oxidation numbers:
- For the lowest oxidation number: ‘oxide (and the element with the prefix hypo- and the suffix -oso)’. For instance: anhydride hypochlorous (Cl2OR).
- For the oxidation number that follows: ‘oxide (and the element with the suffix -oso). For instance: chlorous anhydride (Cl2OR3).
- For the following oxidation number: ‘oxide (and the element with the built-in suffix’ ico ‘)’. For instance: chloric anhydride (Cl2OR5).
- For the highest oxidation number: ‘oxide (and the element with the prefix’ per ‘and the suffix’ ico ‘)’. For instance: perchloric oxide (Cl2OR7).
- The systematic nomenclature
This nomenclature is simpler than traditional, and the oxide is named by writing the word ‘oxide of’ and then the name of the element, but writing before each of them the prefix that corresponds to the number of atoms it has in that chemical compound.
The mono- prefix is for a single atom, the di- prefix for two, the triple- for three, the tetra- prefix for four, the penta- prefix for five, the hexa- prefix for six, the hepta- prefix for seven and the prefix octa- for eight.
This group includes, for example, the dicobre monoxide (Cu2OR), the dialuminium trioxide (Al2OR3), the carbon dioxide (CO2), or the difluorine monoxide (F2OR).
- Stock nomenclature
The word “oxide” is written, followed by the name of the metal and the oxidation number with which it works, in parentheses and in Roman numerals. Analogously to the traditional nomenclature, it will be written chlorine (I) oxide for hypochlorous oxide (Cl2OR), chlorine (III) oxide for chlorous oxide (Cl2OR3), chlorine oxide (V) for chloric oxide (Cl2OR5), and chlorine oxide (VII) for perchloric oxide (Cl2OR7).
[ad_2]
