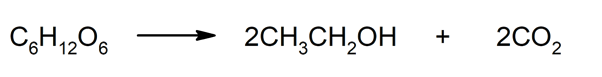Obtaining the ethyl alcohol o Ethanol is produced from two possible sources; A greater percentage of this manufacture is obtained from the fermentation of the sugars contained in some plants such as sugar cane.
But it is not only possible to obtain ethyl alcohol from the sucrose of sugar cane, you can also obtain this compound from the starch of corn and the cellulose of the woods of citrus trees. One of the Applications of the ethyl alcohol obtained from this fermentation is that it is mixed with gasoline and used as fuel.
On the other hand, and for industrial use, this compound is achieved by catalytic hydration of ethylene. The latter (which comes from ethane or oil) is a colorless gas that, mixed with sulfuric acid as a catalyst, produces ethyl alcohol. As a result of this synthesis, ethanol is obtained with water. Later its purification is necessary.
The procurement processes of ethyl alcohol vary slightly depending on the use to which the alcohol is going to be given, but in essence all these processes have the same chemical principles.
Obtaining ethanol from sugar cane

- Fermentation. The process consists of fermenting (with the use of yeasts) the sugar cane molasses. In this way fermented must is obtained. The way to extract the alcohol from this must is through distillation stages.
This fermentation produces chemical changes in the sugar through the use of certain microorganisms (yeasts) that can transform the sugars into ethanol (CH3CHtwoOH) and carbon dioxide (COtwo). The most used for this type of microorganisms is the Saccharomyces Cerevisiae, better known as brewer’s yeast.
Compounds that are necessary for its survival are added to this brewer’s yeast (for example, sulfuric acid, penicillin, ammonium phosphate, zinc sulfate, manganese sulfate, and magnesium sulfate). Thanks to this process, from one molecule of glucose, two (2) molecules of alcohol are obtained.

- Obtaining clean wine. Subsequently, plate and nozzle centrifuges are used to extract the yeast. This produces the separation on the one hand of the yeasts (with a creamy consistency that can be reused for another fermentation if it is subjected to adequate nutrition and acclimatization) and on the other the must without yeasts that receives the name of clean wine.
- Distillation column. When the clean wine enters the distillation columns, two products are obtained; stillage and phlegm. The stillage is composed of potassium, alcohols (in small quantities), organic acids and aldehydes, while the phlegm has a mixture of alcohols. The latter will then be purified in columns like distillers but which are called purifiers.
- The purification columns. These scrubbers achieve the separation of different alcohols from compounds such as esters, aldehydes and ketones. These substances give ethyl alcohol a bad taste, which is why they are called “bad taste alcohol”.
- The retrogradation process. Thanks to the retrogradation process, the phlegm with the alcohols of interest, called “bad taste alcohols” return to the column and concentrate the purified phlegm. This phlegm plays an important role in the rectifier column; further concentrate the cleaned alcohols.
- Rectifier column. This last rectifying column will finally divide the different alcohols. In the lower part will be the water and the higher alcohols; bad taste alcohols and isopropyl will remain in the middle part. Finally, in the upper part of the column, the ethyl alcohol of good taste will be extracted with a percentage around 96 °.