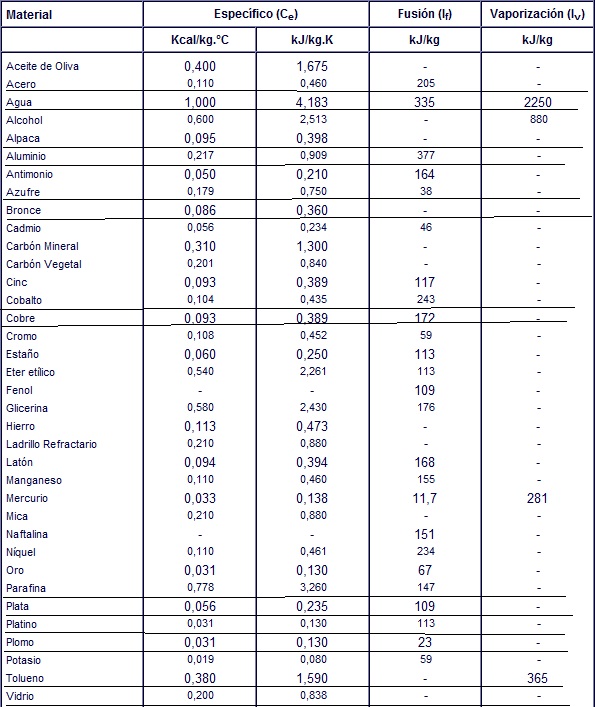
Specific heat, sensible heat, and latent heat are physical quantities:
- Specific heat. It is the amount of heat that must be supplied to a unit of mass of a substance to raise its temperature by one unit. That amount varies depending on the temperature the substance is in before heat is applied to it. For example, it takes one calorie (lime) to increase the temperature of 1 gram of water that was initially at room temperature by one degree Celsius, but it takes only 0.5 calories to increase the temperature of ice that was at room temperature by one degree Celsius. initially at -5 ° C.
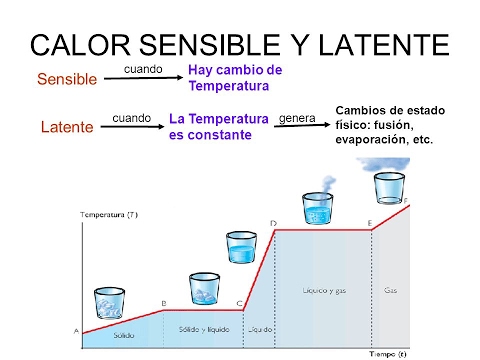
Specific heat also depends on atmospheric pressure. The same substance at a lower pressure has a higher specific heat, this is considering that the volume of the container where the substance is contained is constant. The examples below are valid for a temperature of 25ºC and a pressure of 1 atmosphere. - Sensible heat. It is the amount of heat that a body can receive without affecting its molecular structure. If the molecular structure does not change, the state of aggregation (solid, liquid, gas) does not change. Since the molecular structure does not change, a change in temperature is observed. Therefore, it is called sensible heat.
- Latent heat. It is the energy (in the form of heat) necessary for a substance to change phase (state of aggregation). If the change is from solid to liquid, it is called heat of fusion. If the change is from liquid to gaseous, it is called the heat of vaporization. When heat is applied to a substance that has reached the temperature where it changes state, it is impossible for the temperature to increase during the phase change. When it reaches that temperature (which varies depending on each substance), it simply changes state. In this case, the heat is used to change the phase and not to increase the temperature of the substance. For example, if you continue to apply heat to boiling water, it will never exceed 100 ° C. Depending on the substance, latent heat can usually be measured in calories per gram (cal / g) or in kilojoules per kilogram (kJ / kg).
Examples of specific heat
- Water (in liquid state): 1 calorie per gram to increase 1 ° C.
- Aluminum: 0.215 calories per gram and per degrees Celsius (cal / g ° C)
- Beryllium: 0.436 cal / g ° C
- Cadmium: 0.055 cal / g ° C
- 0.0924 cal / g ° C
- Glycerin: 0.58 cal / g ° C
- Gold: 0.0308 cal / g ° C
- Iron: 0.107 cal / g ° C
- Lead: 0.0305 cal / g ° C
- Silicon: 0.168 cal / g ° C
- Silver: 0.056 cal / g ° C
- Potassium: 0.18 cal / g ° C
- Toluene: 0.2 cal / g ° C
- Glass: 0.17 cal / g ° C
- Marble: 0.21 cal / g ° C
- Wood: 0.41 cal / g ° C
- Ethyl alcohol: 0.58 cal / g ° C
- Mercury: 0.033 cal / g ° C
- Olive oil: 0.47 cal / g ° C
- Sand: 0.2 cal / g ° C
Examples of sensible heat
- Apply heat to the water until its temperature is between 1 and 100 ° C.
- Apply heat to the tin until its temperature is below 240 ° C.
- Apply heat to the lead until its temperature is below 327 ° C.
- Apply heat to the zinc until its temperature is below 420 ° C.
- Apply heat to the aluminum until its temperature is below 660 ° C.
- Apply heat to the bronze until its temperature is below 880 ° C.
- Apply heat to nickel until its temperature is below 1450 ° C.
Examples of latent heat
- Water. Latent heat of fusion: 80 calories per gram (cal / g) (it takes 80 calories for one gram of ice at 0 ° C to convert to water), latent heat of vaporization: 540 calories per gram (it takes 540 calories to that a gram of water at 100 ° C turns into steam).
- Steel. Latent heat of fusion: 25 kJ / kg.
- Aluminum. Latent Heat of Fusion: 322-394 x103 J / kg; latent heat of vaporization: 9220 x103 J / kg.
- Sulfur. Latent heat of fusion: 38 kJ / kg; latent heat of vaporization: 326 kJ / kg.
- Cobalt. Latent heat of fusion: 263 J / g.
- Copper. Latent Heat of Fusion: 214 x103 J / kg; latent heat of vaporization: 5410 x103 J / kg.
- Tin. Latent Heat of Fusion: 59 x103 J / kg.
- Phenol. Latent heat of fusion: 122.6 kJ / mol.
- Iron. Latent Heat of Fusion: 293 x103 J / kg; latent heat of vaporization: 6300 x103 J / kg.
- Magnesium. Latent heat of fusion: 362 J / g.
- Mercury. Latent Heat of Fusion: 11.73 x103 J / kg; latent heat of vaporization: 285 x103 J / kg.
- Nickel. Latent heat of fusion: 292 J / g.
- Silver. Latent heat of fusion: 88.3 kJ / kg.
- Lead. Latent Heat of Fusion: 22.5 x103 J / kg; latent heat of vaporization: 880 x103 J / kg.
- Oxygen. Latent heat of fusion: 13.8 kJ / kg.
- Gold. Latent heat of fusion: 64.5 kJ / kg.
- Zinc. Latent heat of fusion: 111 J / g.
