Mixtures of gaseous substances They are some of the easiest to achieve, since it is very possible that substances that are in this state of aggregation are mixed. For example: Neon, argon and xenon mixture, aerosol insecticide, air and helium.
Practically all gases can be combined without limits, of course conditioned by some chemical and physical aspects and fundamentally related to the safety of the user who handles them. Like the different types of mixtures that are established between substances, gas mixtures also have properties that are unique to them.
The study of fizzy mixes It is usually as useful as that of gases in their pure state: the same knowledge about the air that is present in the atmosphere would be impossible if it were not for the knowledge about the proportions and behaviors of mixed gases.
In this way, it is essential to know some characteristics of gas mixtures, such as the property of partial pressure (that exerted by each of the gases within the mixture) and that of the mole fraction (ratio of the number of moles of a gaseous component to that of the total of the gas mixture). The moles express the amount of gas in the mixture.
The Dalton’s Law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each of the individual gases that participate in it (this is subject to the gases not reacting with each other). The partial pressure is understood here as that which each of the gases would exert if it were found alone in the same container and under the same temperature conditions. A) Yes:
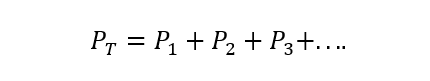
Where PT is the total pressure and Pone, Ptwo And p3 are the partial pressures of the hypothetical gases 1, 2 and 3 in the gas mixture.
Using Dalton’s Law, an expression was developed to calculate the partial pressure of a gas in a gaseous mixture if we know its total pressure and its mole fraction.
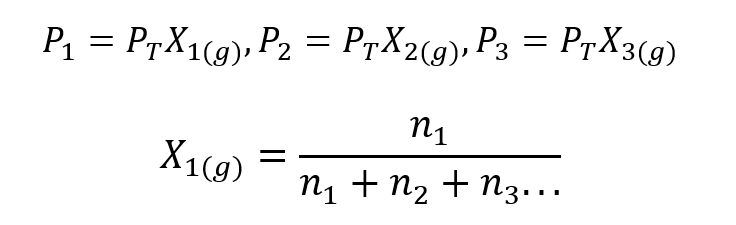
Where Xone, Xtwo Y X3 are the mole fractions of the hypothetical gases 1, 2, and 3 in the gas mixture, and none, ntwo Y n3 are the amounts of substance of each of these gases in the same mixture.
One of the main properties of the components in mixtures is the concentration, which can be expressed in different units. In the case of gas mixtures, the concentration of its components can be expressed in ppm (parts per million), a unit referred to its volume. That is, a certain amount of ppm of a component in a gas mixture is the ratio of the volume of that certain amount to the volume of each million units of mixture.
It is important to note that ppm of a gas depend on the temperature and pressure of the gas. For this reason, to calculate the ppm of a gas, the normal conditions of pressure and temperature (CNPT) are usually used, which give the normal state to 0 degrees Celsius of temperature, and 1013 hectopascals (1 bar) of pressure. If these conditions are not used, you must specify which ones will be used.
Examples of gas mixtures
The following list contains gas mixtures, specifying the elements that appear in the mixture:
- Air (mixture of 21% oxygen and 79% nitrogen, plus other gases in small proportions)
- Cronigón (mixture of 99% argon and 1% oxygen)
- Trimix (mixture of oxygen, nitrogen and helium)
- Neon, argon and xenon mix
- Blend of 85% methane, 9% ethane, 4% propane, and 2% butane.
- Sulfur hexafluoride and air
- Aerosol insecticide
- Air and helium
- Nitrox (air mixture, enriched in oxygen and nitrogen)
