Chemistry distinguishes between two types of molecules that make up matter, according to the type of atoms on which their structures are based: organic molecules and inorganic molecules.
The fundamental difference between both types of molecules (and between the substances that are composed of them) is based, more than anything, on the presence of carbon atoms (C) forming covalent bonds with other carbon atoms and with hydrogen atoms (H), as well as with other frequent elements such as oxygen (O), nitrogen (N), Sulfur (S), Phosphorus (P) and some metals. Molecules that have this carbon-based structure are known as organic molecules and are essential for life.
On the other hand, molecules whose structure is not based on carbon (it does not mean that they cannot contain carbon), are called inorganic.
Organic molecules
One of the main features of most organic substances is their combustibility, that is, their ability to burn and lose or vary their original structure, as is the case of hydrocarbons that make up fossil fuels. On the other hand, some are soluble in organic solvents like gasoline, while others are soluble in water. They tend to have lower melting and boiling points than inorganic compounds.
There are two types of organic substances, depending on their origin:
- Natural organic molecules. Those that are synthesized by living beings and that constitute the fundamental building blocks for the functioning and growth of their bodies. They are known as biomolecules. For instance: glucose, cholesterol, collagen. There are also natural organic molecules that are part of oil, originating thousands of years ago.
- Artificial organic molecules. They owe their origin to the hand of man, since they do not exist in nature as such, so they are synthesized in laboratories and industries. It is the case of plastics, for instance.
It should be noted that broadly there are several types of organic molecules that make up the body of living beings: protein, lipids, carbohydrates, nucleotides and small molecules.
Examples of organic molecules
- Glucose (C6H12OR6). One of the main sugars (carbohydrates) that serve as the basis for the construction of the various organic polymers (energy reserve or structural function). Animals obtain their vital energy (respiration) from biochemical processing.
- Cellulose (C6H10OR5). It is an essential biopolymer for plant life and the most abundant biomolecule on the planet. Without it, it would be impossible to build the cell wall of plant cells, so it is a molecule with irreplaceable structural functions.
- Fructose (C6H12OR6). It is a monosaccharide present in fruits, vegetables and honey, it has the same formula but different structure than glucose (it is its isomer). Together with the latter, it forms sucrose or common table sugar.
- Formic acid (CH2OR2). It is the simplest organic acid that exists, used by ants and bees as an irritant for their defense mechanisms. It is also secreted by nettles and other stinging plants, and is part of the compounds that make up honey.
- Methane (CH4). It is the simplest alkane hydrocarbon of all, whose gaseous form is colorless, odorless and insoluble in water. It is the majority component of natural gas and a frequent product of animal digestion processes.
- Collagen. It is a protein necessary for the formation of fibers, common to all animals and that makes up bones, tendons and skin, which adds up to 25% of the total proteins of the mammalian body.
- Benzene (C6H6). It is an aromatic hydrocarbon composed of six carbon atoms located at the vertices of a perfect hexagon, which are linked by a typical covalent bond and also by an electronic cloud of the pi type (). It is a colorless liquid with a highly flammable sweet aroma.
- DNA (deoxyribonucleic acid). It is a polymer of nucleotides and the basic molecule of the genetic material of living beings, whose instructions allow the replication of all the material necessary for its creation, operation and eventual reproduction. Without DNA, the transmission of hereditary information would be impossible.
- RNA (ribonucleic acid). It is the other essential molecule in the synthesis of proteins and substances that make up living beings. Formed by a chain of ribonucleotides, it relies on DNA for the execution and reproduction of the genetic code, key in cell division and in the constitution of all complex life forms.
- Cholesterol. It is a lipid present in the body tissues and blood plasma of vertebrates, essential in the constitution of the plasma membrane of cells, despite the fact that its very high levels in the blood can lead to problems in blood circulation.

Inorganic Molecules
The inorganic molecules They are not based on carbon but other various elements. They are formed as a result of different physical and chemical processes such as: fusion, electrolysis, the action of solar energy. For instance: ozone, calcium oxide, helium.
The dividing line between organic and inorganic molecules it has often been questioned and considered arbitrary, since many inorganic substances contain carbon and hydrogen. However, the established rule is that all organic molecules are based on carbon, but not all carbon molecules are organic.
Examples of inorganic molecules

- Carbon monoxide (CO). Despite consisting of just one carbon and one oxygen atom, it is an inorganic molecule and a highly toxic environmental pollutant, that is, it is incompatible with the majority of known living beings.
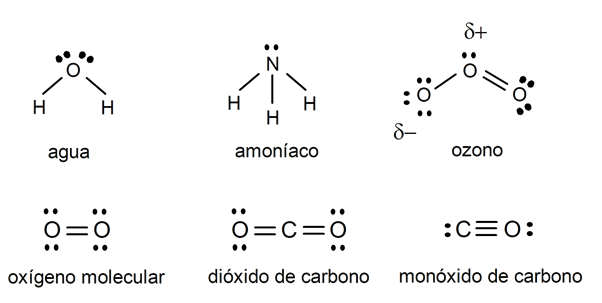
- The water (H2OR). Although essential to life and perhaps one of the most widely known and abundant molecules, water is inorganic. It is capable of containing living beings in its interior, such as fish in the seas, lakes and rivers. On the other hand, living beings contain it inside, but it is not properly a biomolecule.
- Ammonia (NH3). It is a colorless gas with a repulsive odor, the presence of which in living organisms is toxic and lethal, despite the fact that it is a by-product of many biological processes. That is why it is excreted from their bodies, in the urine, for example.
- Sodium chloride (NaCl). It is the molecule of common salt, soluble in water and present in living organisms, which ingest it through their diet and dispose of the excess through various metabolic processes.
- Calcium oxide (CaO). Known as “lime” or “quicklime”, it comes from limestone rocks and has long been used in history in construction work or in the manufacture of greek fire.
- Ozone (O3). It is a substance very present in the upper part of the atmosphere (the ozone layer), whose special conditions allow it to exist, since normally its bonds decay and recover the diatomic form (O2). It is used to purify water, but in large quantities it can be irritating and slightly toxic.
- Ferric oxide (Fe2OR3). Common iron oxide is a metal widely used in various human industries. It is reddish in color and is not a good conductor of electricity. It is stable to heat and dissolves easily in acids, giving rise to other compounds.
- Helium (He). It is a noble gas (along with argon, neon, xenon and krypton) of very low or null chemical reactivity, which exists in its monatomic form.
- Carbon dioxide (CO2). It is the molecule resulting from the respiration of aerobic living beings, which expel it. And it is necessary for the photosynthesis of plants, which take it from the air. It is a vital substance for life, but it is not part of organic molecules, despite having a carbon atom.
- Sodium hydroxide (NaOH). Known as “caustic soda,” it is in the form of odorless white crystals. It is a strong base, that is, a highly desiccant substance, which reacts exothermically (generating heat) when dissolved in water. In contact with organic substances it generates corrosion damage.