Salts are chemical compounds made up of cations and anions joined by ionic bonding. There are different types of neutral salts, depending on the cations and anions that form them and the chemical reactions from which they are obtained.
Neutral oxysalts are a type of salt that arises from the reaction of a base or hydroxide with an oxo acid or oxacid. For instance: calcium nitrate (Ca2NO3).
In the formation of a neutral oxisal, all the hydrogen ions of the oxacid are replaced by cations of the hydroxide and water is also formed in the reaction:
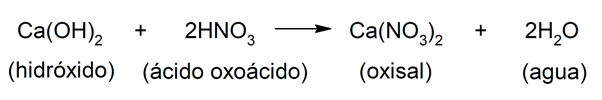
According to Stock nomenclature, the oxysalts are named by putting the name of their anion in the following way: the ending of the name of the anion that corresponds to the oxoacid is changed (-oso or -ico) from which the salt comes, by the ending -ito or -ato, in that order. Then you put “of” and the name of the cation. For example: nitrtied calcium (Ca2NO3), the anion comes from nitric acidico (HNO3).
When the oxacid retains at least one of the hydrogens, an acid salt is obtained. For instance: sodium bicarbonate (NaHCO3), only one hydrogen ion of carbonic acid (H2CO3) by the sodium cation (Na+).
There are also binary salts or neutral hydracides. They are made up of a metal and a non-metal. They can be obtained by the reaction between a hydroxide and a hydric acid.

According to the Stock nomenclature, they are named by putting the name of their anion in the form: the ending is changed -hydric of the anion corresponding to the hydracid by the termination –aurochs. Then it is put “of” followed by the name of the cation. For instance: chloraurochs sodium (NaCl), the anion comes from chloric acidhydric (HCl).
Examples of neutral salts
- NaCl: Sodium chloride
- CaCl2: Calcium chloride
- Case4: Calcium sulfate
- KNO3: Potassium nitrate
- CaF2: Calcium fluoride
- MgSO4: Magnesium sulphate
- To the2(SW4)3: Aluminum sulfate
- Faith (NO3)3: Iron (III) nitrate
- AlCl3: Aluminum chloride
- K2S: Potassium sulfide
- CuCl: Copper (I) chloride
- FeCl2: Iron (II) chloride
- CaS: Calcium sulfide
- CrS: Chromium (II) sulfide
- Faith3N2: Iron (II) nitride
- Na3N: Sodium nitride
- B2S3: Boron sulfide
- CaBr2: Calcium bromide
- LiF: Lithium Fluoride
- (NH4)2SW4: Ammonium sulphate
- BaCl2: Barium chloride
- LiCl: Lithium chloride
- KCl: Potassium chloride
- AgCl: Silver chloride
- AuPO4: Gold (III) phosphate
- To the2(SW4)3: Aluminum sulfate
- Mg3(PO4)2: Magnesium phosphate
- Ca (ClO2)2: Calcium chlorite
- FeSO4: Iron (II) sulfate
- MgS: Magnesium sulfide
