The chemistry It is the science that studies the composition and the transformations that can occur to matter, in any of its forms. One of the most important areas of study in chemistry is that of gases.
The gas concept It was established by the Belgian chemist Jan van Helmont. To explain the behavior of gases, different mathematical equations were developed using statistical tools. However, it was necessary to simplify and modify these equations because they did not work for all types of gases, so different gas models were defined (ideal gas and real gas, among other intermediate approaches). For instance: nitrogen, helium, methane.
In this sense, three laws were established to generally relate the volume, temperature and pressure of gases:
- Boyle-Mariotte law. It states that the pressure of a gas is inversely proportional to its volume at constant temperature.
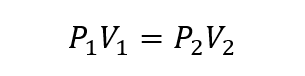
- Charles Law. It establishes that the volume that a quantity of gas occupies is directly proportional to its temperature at a given pressure.
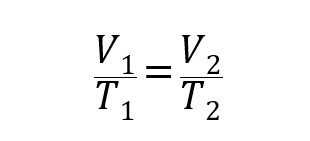
- Gay-Lussac Law. It states that the pressure of a gas is directly proportional to its temperature at constant volume.
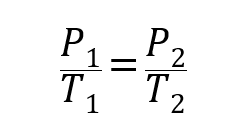
Where P1, V1 and T1 are the initial pressure, volume and temperature of the gas respectively, and P2, V2 and T2 are the finals.
Thus, relating the three laws, we obtain the General Gas Law,
PV / T = C where C is a constant that depends on the amount of gas.
Ideal gas examples
The ideal gas it is a theoretical model that represents a gas that does not really exist. It is a tool to facilitate a large number of mathematical calculations, as it greatly simplifies the complex behavior of a gas. This gas is considered to be made up of particles that neither attract nor repel each other and whose collisions are absolutely elastic. It is a model that fails if the gas is subjected to high pressures and low temperatures.
The general equation ideal gas results from the combination of the laws of Boyle-Mariotte, Charles and Gay Lussac with Avogadro’s law. Avogadro’s law states that if different gaseous substances are contained in equal volumes and subjected to the same pressure and temperature, then they have the same number of particles. Thus, the ideal gas equation of state is:
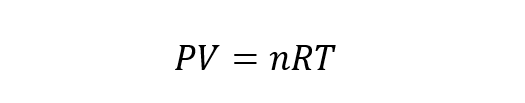
Where n is the number of moles of the gas and R is the gas constant equal to 8.314 J / Kmol.
It is not possible to make a specific list of ideal gases since it is a hypothetical gas. e can list a set of gases (including noble gases) whose treatment can be approximated to that of ideal gases, because the characteristics are similar, as long as the pressure and temperature conditions are normal.
- Nitrogen (N2)
- Oxygen (O2)
- Hydrogen (H2)
- Carbon dioxide (CO2)
- Helium (He)
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
- Radon (Rn)
The real gases They are those that have a thermodynamic behavior and that is why they do not follow the same equation of state as ideal gases. In high pressure and low temperature, gases must inevitably be considered as real, because in that case the interactions between their particles increase.
The substantial difference between the ideal gas and the real gas is that the latter cannot be compressed indefinitely but its compression capacity is relative to the pressure and temperature levels.
Different equations have been developed to explain the behavior of real gases. One of the most important is the one provided by Van der Waals in 1873, which must be applied under high pressure conditions. The Van der Waals equation is represented as:
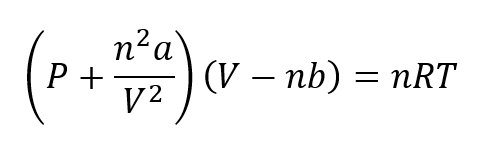
Where to and b they are constants referred to the nature of each gas.
The following list shows some examples of real gases, although you can also add those that have already been listed as ideal gases, but this time in a context of high pressure and / or low temperature.
- Ammonia (NH3)
- Methane (CH4)
- Ethane (CH3CH3)
- Ethene (CH2CH2)
- Propane (CH3CH2CH3)
- Butane (CH3CH2CH2CH3)
