They are known as solid to objects that occur in this state of matter. Together with the other two (liquid and gaseous), they make up the three classically recognized possible states. For instance: diamond, iron, sand, silicon.
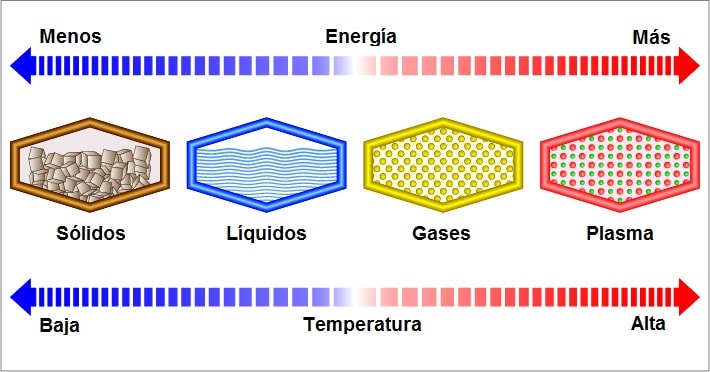
Some incorporate a fourth state, the plasmatic state, only feasible under extremely high temperatures and pressures, in which the impacts between the electrons would be very violent, which is why they would tend to separate from the nucleus.
At solid state, the particles that make up matter are held together by very strong attractive forces, which makes them stay fixed and can only vibrate in place.
In liquids, the interparticle attraction is lower, the particles can vibrate but also move and collide with each other. In gases, there is almost no interparticle attraction: the particles are well separated and can move in all directions quickly.
Characteristics of solids
Solids are characterized by certain fundamental properties: they have a constant shape and volume and are not compressible, that is, they cannot be “shrunk” by squeezing or squashing them. However, many of them are deformable or have other mechanical properties (for example, they can be elastic).
On the other hand, they increase their volume when they heat up and decrease it when they cool down; These phenomena are known as expansion and contraction, respectively. They often form structures of a certain regularity, such as crystalline ones; this regularity is perceived only by microscopic observation.
They can also be amorphous. They are generally rather rigid and of high density, although some solids (especially synthetics) have low density, including certain expanded polystyrenes (Styrofoam).
Changes in the states of the matter
Due to pressure and temperature changes, solids can change state. The transition from solid to liquid is known as fusion; the one from solid to gas, as sublimation. In turn, the gas can be transformed into a solid by inverse sublimation and the liquid does the same by solidification.
The temperature at which a solid passes into a liquid state is known as the melting temperature, and it is one of the properties that characterizes it, as well as being important when considering its possible uses.
Examples of solids
- Table salt
- Diamond
- Sulfur
- Quartz
- Mica
- Iron
- Table sugar
- Magnetite
- Ilita
- Kaolin
- Sand
- Graphite
- Obsidian
- Feldspar
- Cast
- Borosilicate
- Mineral carbon
- Silicon
- Limonite
- Chalcopyrite
