[ad_1]
The organic chemistry or carbon chemistry deals with the study of organic compounds, which are those that are composed of carbon and hydrogen atoms, often combined with others such as oxygen, nitrogen, phosphorus, sulfur, iron, magnesium, chlorine and others. For instance: ethanol, ethylamine, nitroethane.
Organic chemistry pays special attention to the synthesis and degradation processes of this type of substances, which are those that regulate most vital processes.
The metabolism of carbohydrates or sugars, lipids, fats, proteins, nucleic acids, vitamins and hormones, for example, is governed by a complex network of chemical reactions between organic compounds and between organic and inorganic compounds, such as addition, substitution, rearrangement or elimination reactions.
Characteristic reactions in organic chemistry
In addition, there are characteristic reactions in organic chemistry, such as the combustion of hydrocarbons, the saponification or transesterification of fats, the polymerization of different molecules, condensation reactions of aromatic compounds, diazotization reactions, and many others. Some examples of these reactions are:
- Addition reactions. They occur by adding two chemical compounds to the double bond of an unsaturated molecule.
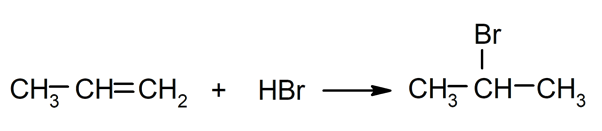
- Substitution reactions. They occur when an atom or a set of atoms in an organic compound is replaced by another.
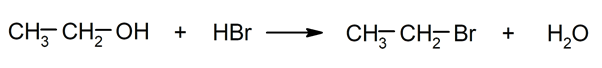
- Transposition reactions. They occur when a reorganization of the structure of the initial molecule occurs to originate another molecule.

- Elimination reactions. They occur when there is a loss of atoms or groups of atoms in a molecule. As a result, compounds with double bonds or cyclic compounds can be obtained.
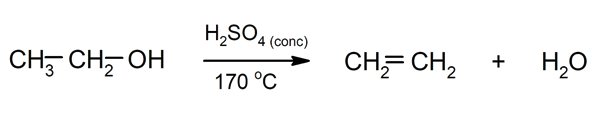
- Combustion reactions of alkanes. They are rapid oxidations that release water, carbon dioxide, and energy in the form of light or heat.
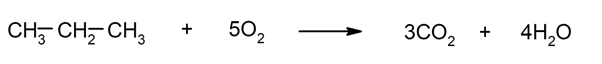
- Saponification reactions. They occur when a fatty acid reacts with a base to obtain soap and glycerin.
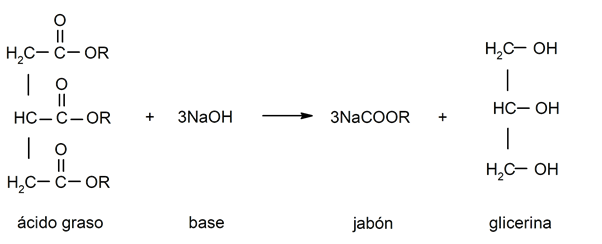
- Aromatic compound condensation reactions. They occur when two molecules combine to generate a resulting molecule.

Organic chemistry is fully integrated into our daily work and also into many industrial processes. In the day to day, for example, when making a cake or a pizza, what we are achieving is the fermentation of the carbohydrates contained in the flour: when the dough rises, carbonic gas is formed, which is what gives it the aeration of baked goods.
The production of medicines, paints and varnishes, pesticides, plastics, food preservatives, cosmetics, among many others, is based on organic reactions of different types (often quite complex).
Study of organic chemistry
The concept of “organic chemistry” was introduced in 1807 by Berzelius, to refer to compounds that came from natural resources. At that time it used to be thought that life-related compounds had a “vital” component that made them different from inorganic ones. Furthermore, it was considered that it was not feasible to prepare an organic compound in the laboratory.
However, in 1828 Friederich Wöhler succeeded in converting lead cyanate to urea by treatment with aqueous ammonia. In this way it was possible to obtain a typically organic product from an inorganic salt. There are already more than ten million organic compounds that man has managed to synthesize and take advantage of.
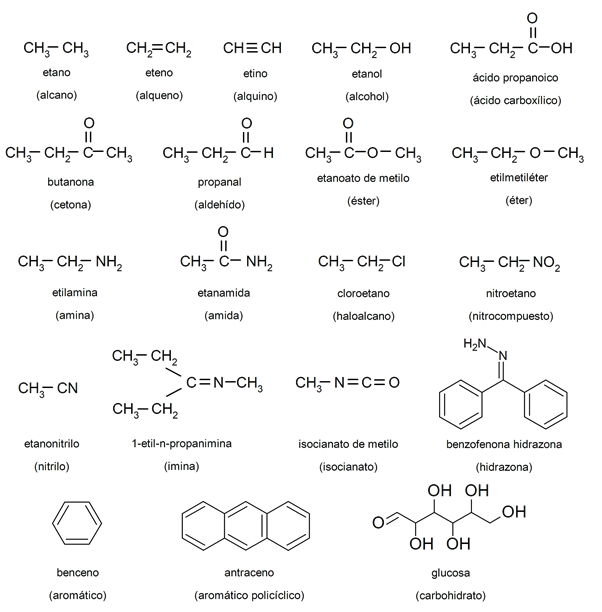
To facilitate the study of organic chemistry, a large number of organic compounds have been defined that have been identified according to their functional groups such as alkanes, alkenes, alkynes, alcohols, aldehydes, carboxylic acids, epoxides, haloalkanes, hydrazones, imides, imines, isocyanates , isonitriles, isothiocyanates, ketones, nitriles, nitroso compounds, organophosphates, oximes, peroxides, phosphonates, pyridine derivatives, sulfones, sulfonates, sulfoxides, thiocyanates and isothiocyanates, thioesters, thioketones, thiols.
Examples of organic chemistry
[ad_2]
