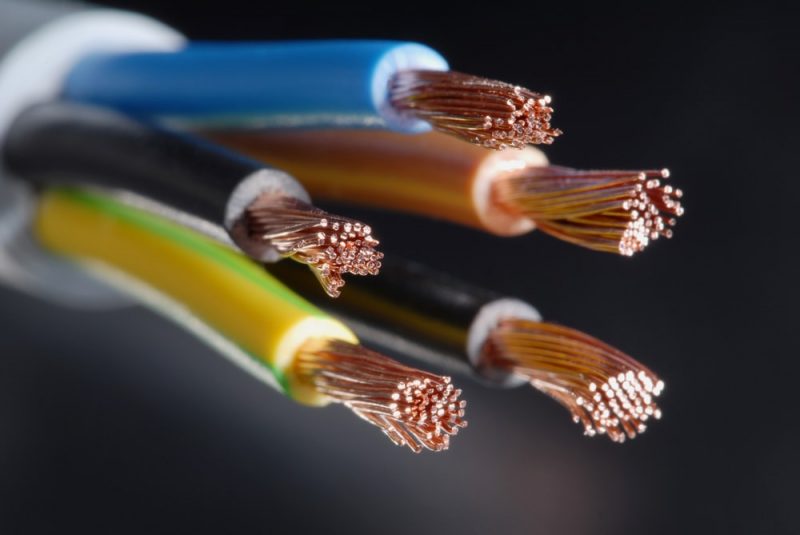
The conductive materials They are those that offer little resistance to the passage of electricity. Electrons can circulate freely through material because they are loosely bound to atoms and can therefore conduct electricity. For example: aluminum, bronze, nickel, gold.
Although all materials allow the conduction of electric current to some degree, those who do it best are recognized as conductors, while materials that do not allow electricity to pass through are insulators. There is an average level between the two made up of semiconductor materials, which behave as insulators in certain circumstances but their conductivity can be altered according to the conditions to which they are subjected.
Chemically, the process that occurs with conductive materials is that some electrons pass freely from one atom to another through a process of potential difference between the ends of the conductor. This movement of the electrons is the electric current.
The conductors, then, are those that have a large number of free electrons that move through the material, transmitting the charge more easily from one object to another. To describe these materials, on many occasions a comparison is made with a pipe through which a strong flow of water passes.
The mechanisms of conductivity are not identical in the three states of matter. In the case of liquids, conductivity is related to the presence of salts in solution, while in solids, conductivity is related to valence bands and the formation of an electron cloud.
Types of conductors
According to the way in which conduction is carried out and based, materials of this type are usually classified as follows:
- Metallic conductors. They are those that have an electronic conduction, since the carriers of the charges are free electrons. This occurs because metals and alloys belong to this group.
- Electrolytic conductors. They are those that have an ionic type conduction, where the substances totally or partially dissociate, forming positive or negative ions, which are the charge carriers. Here the passage of electric current occurs in line with a displacement of matter and with a chemical reaction.
- Gaseous conductive materials. They are those gases that have been ionized (have lost or gained electrons) and thus have acquired the ability to conduct electricity. Although they are not used frequently, air is a gas and under certain conditions it is a great conductor of electricity, which is evidenced by lightning and electrical discharges of this type.
Examples of conductive materials
| Pure silver | Gallium | |
| Tungsten | Hardened copper (**) | Nickel |
| Iron | Aluminum | Graphite |
| Cast iron | Pure zinc | Tantalum |
| Copper | Bronze with phosphor | Bronze |
| Gold | Brass | Galvanized Metal |
| Ionized air |
Steel
Pure silver : Element known to be the best conductor of electricity.(**)
Hardened copper
: Although not as effective as silver, it is cheaper and therefore more widely used.
