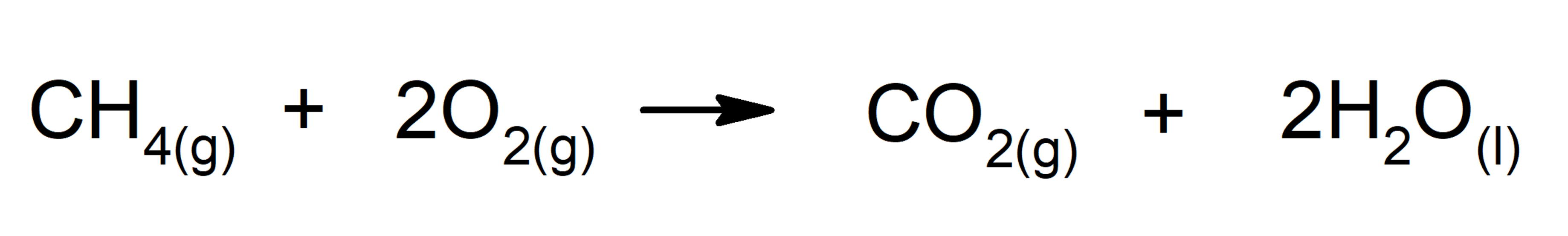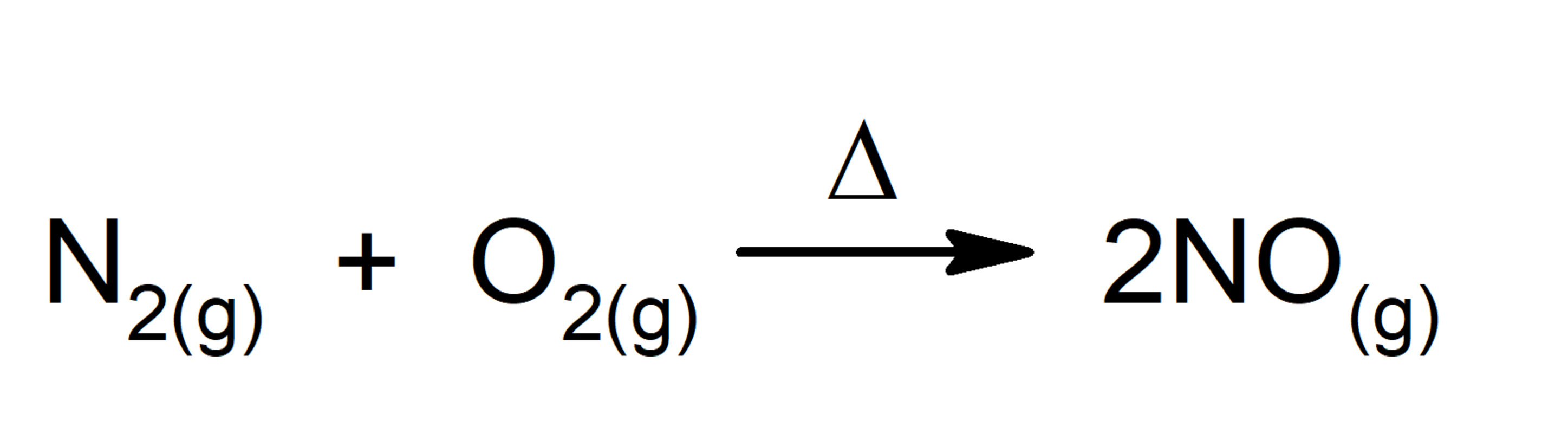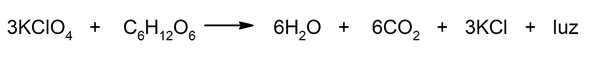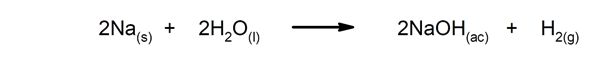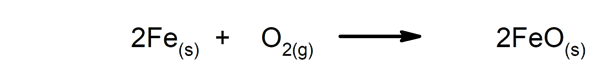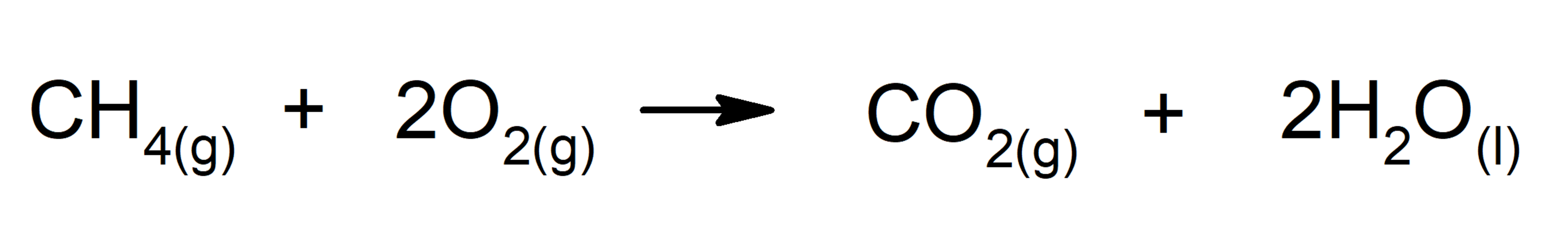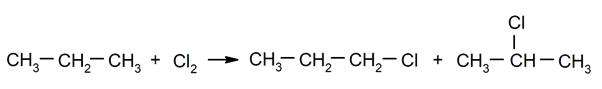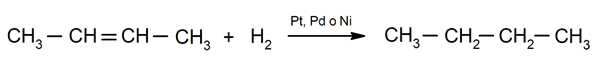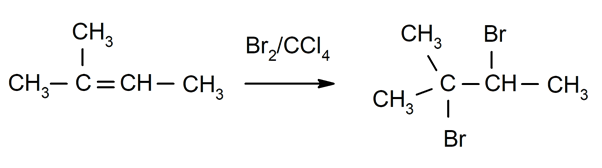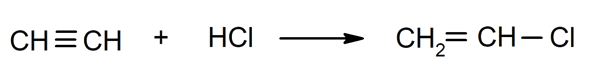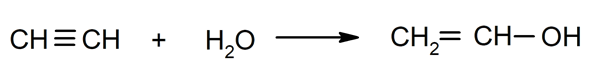The chemical changes (or chemical reactions) are modifications that substances undergo and that turn them into different ones. This is because they undergo a modification in their nature. They are changes that a substance undergoes and that involve breaking and the formation of chemical bonds to generate a new substance.
Chemical changes differ from Physical changes since in physicists there is no transformation in nature, but there is simply a change of state, volume or shape.
For example, when you put water in a pot and it boils, goes from a liquid to a gaseous state. This process is a reversible physical change, that is, the water vapor can return to being liquid when the conditions of pressure or temperature change.
The chemical changes, then, they are not always reversiblewhile physicists generally are. Furthermore, chemical changes can be analyzed from two points of view:
- Macroscopic. It is that change in which the formation of new substances from others is analyzed.
- Molecular. It is that process in which the breaking of bonds or the reorganization of atoms or ions to form new structures is analyzed.
Types of chemical changes
Chemical changes can be classified depending on the type of substances that react (inorganic or organic). In addition, reactions can be defined according to several criteria, some of which are:
Inorganic reactions
- Reactions that release energy in the form of heat. For instance:

- Reactions that absorb energy in the form of heat. For instance:

- Reactions that release energy in the form of light. For instance:

- Reactions that absorb energy in the form of light. For instance:

- Quick reactions. For instance:

- Slow reactions For instance:

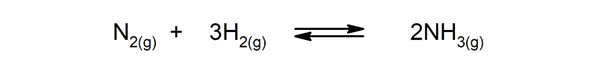
- Reversible reactions. For instance:

- Irreversible reactions. For instance:

- Acid-base reactions. For instance:

Organic reactions
They are classified according to many factors such as the type of compound that reacts, the type of reaction it undergoes, the conditions under which the reaction occurs, and many more. Some organic reactions are:
- Combustion of alkanes. For instance:

- Halogenation of alkanes. For instance:

- Hydrogenation of alkenes. For instance:

- Halogenation of alkenes. For instance:

- Halogenation of alcohols. For instance:

- Halogenation of alkynes. For instance:

- Hydration of alkynes. For instance:

Examples of chemical changes
Here are some chemical changes:

- When we burn logs to make a fire, a chemical change occurs. This is because the wood in the logs turns to ash and, in turn, releases some gases, such as carbon dioxide.
- The production of water, as a consequence of the combination of two molecules of hydrogen and one of oxygen.
- The transformation of starch into different types of sugar, when they come into contact with saliva, at the moment in which we digest it.
- When we combine sodium with chlorine and they react, as a consequence common salt is obtained, also called sodium chloride.
- In the digestion of food, what we eat is then transformed into the energy we need to live and to carry out different activities, from the basic ones such as walking and breathing, to the more complex ones, such as thinking and working.
- During photosynthesis (a process carried out by plants), solar energy becomes their power source.
- When atoms are transformed into ions.
- Diesel, as a result of the refining processes that oil undergoes.
- When we put a piece of paper in a flame of fire and it burns and turns to ashes.
- Baking a cake mix (it can no longer be returned to its previous state).
- The burning of gunpowder, when we light a firework or when we shoot a weapon.
- When we forget the fruits outside the refrigerator for several days, the bacteria begin to act on them, until they oxidize.
- The transformation of wine into vinegar occurs when bacteria begin to act and transform ethyl alcohol into what is known as acetic acid.
- Cooking a piece of pork on a griddle.
- Ammonia, which is produced from the mixture of nitrogen and hydrogen.
- When the grape ferments (which implies a change in the sugar contained in the fruits) and turns into wine.
- When we breathe, we inhale oxygen, which is then converted into carbon dioxide that we exhale.
- The combustion of gasoline in a motorcycle when it is running.
- When we prepare a fried egg.
- The production of bioethanol for use as a biofuel.