
Is named chemical base to all substances that, when dissolved in water, liberate hydroxyl ions (OH–). For instance: calcium hydroxide, copper hydroxide, zinc hydroxide.
Chemical bases are also known as alkali, because by dissociating and releasing the hydroxyl groups (OH–), the pH of the solutions increases, that is, the solution becomes alkaline. This is contrary to what happens when an acid dissolves, because in that case the pH decreases and the solution becomes acidic.
The bases have a bitter taste characteristic. After dissolving, the resulting solutions conduct the electric current (due to the presence of ions) and are usually caustic and irritating to the skin and other human and animal tissues.
The bases neutralize acids, often forming salts. Alkaline solutions tend to feel slippery or soapy; This happens because they immediately saponify the fats present on the skin’s surface.
In the case of hydroxides, their solubility depends on the metal: those of group (I) are the most soluble in water, on the other hand, the hydroxides of the elements with a degree of oxidation (II) are less soluble and those with a degree of oxidation ( III) or (IV) are almost insoluble. Amines and nucleic acid bases are the most widespread of the organic bases.
Theories that define the bases
There are different theories to define chemical bases:
- The Arrhenius theory. Establishes that a base is a substance that when dissolved in water releases OH ions–. The drawback of this theory is that it is based on the fact that the substance must be in aqueous solution and contain OH– as part of its structure. For this reason, some bases such as ammonia (NH3), which do not have OH–, could not be explained through this theory. For example, according to Arrhenius, basic substances are sodium hydroxide (NaOH) and potassium hydroxide (KOH).
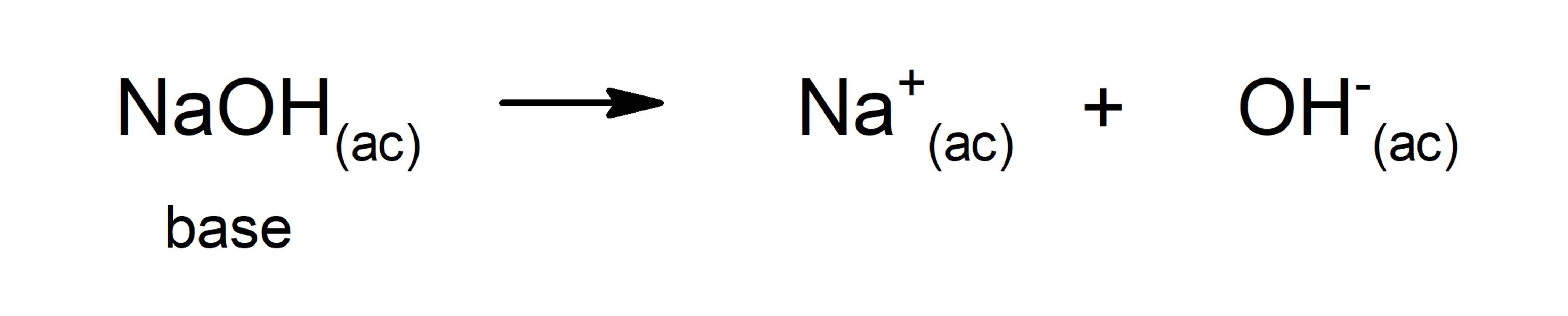
- Brönsted and Lowry’s theory. Define a base as a substance that captures cations H3OR+ (H+) and that it is also involved with its conjugated acid, which is the substance that gives up the H cations+ that captures the base. This theory solves the drawback of Arrhenius’s theory of the need for substances defined as bases to be in an aqueous medium since, according to Brönsted and Lowry, bases are defined in any solvent. For example, some bases defined by Brönsted and Lowry are ammonia (NH3) and disulfide (HS–).
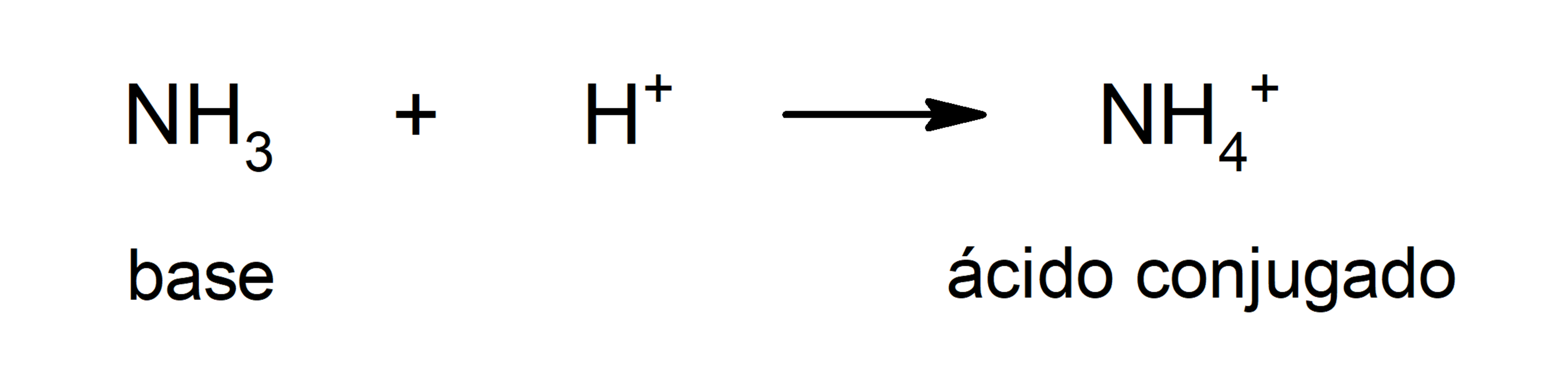
- Lewis theory. Name a base as a substance that can donate a pair of electrons. For example, the OH ion– and the ammonia molecule (NH3) are bases by Lewis’s definition, as they have a nonbonding electron pair that they can donate. An acid-base reaction, according to this theory, can be represented as:

Thus, the OH ion– donates its nonbonding electron pair to the H+ to form a coordinated or dative bond (it is a type of covalent bond where only one of the atoms that form the bond provides the pair of electrons) through which a water molecule is formed.
Uses of the bases
The sodium hydroxide It is widely used in industry: it is called caustic soda. In the manufacture of soap, animal or vegetable fats are used, which are boiled with sodium hydroxide and, in this way, sodium stearate is formed.
Sodium hydroxide is also used in the manufacture of oven cleaners, in the manufacture of paper pulp, and in some household cleaners. Another widely used base is calcium hydroxide, which is the slaked lime used in construction.
Examples of chemical bases
- Calcium hydroxide – Ca (OH) ₂
- Potassium hydroxide – KOK
- Barium hydroxide – Ba (OH) ₂
- Magnesium Hydroxide – Mg (OH) ₂
- Ammonia – NH₃
- Copper (II) hydroxide – Cu (OH) ₂
- Iron (II) hydroxide – Fe (OH) ₂
- Aluminum hydroxide – AI (OH) ₃
- Zinc Hydroxide – Zn (OH) ₂
- Sodium hydroxide – NaOH
- Nickel (II) hydroxide – Ni (OH) ₂
- Detergent soap

