The alkenes They are compounds that contain carbon-carbon double bonds. When these compounds have open chain structures they respond to the molecular formula CnH2n (where n is the number of carbon atoms). Alkenes are also called olefins and correspond to the group of unsaturated hydrocarbons. They are obtained mainly as part of the oil cracking process and by dehydrogenation of alkanes. For instance: ethene, propene, cyclohexene.
They are organic compounds that can be short, medium or long chain; there are also cyclic alkenes or cycloalkenes. By having the carbon-carbon double bond, alkenes have fewer hydrogens than corresponding alkanes with the same number of carbon atoms.
How are alkenes named?
To name the alkenes, the carbon chain that contains the greatest amount of carbon atoms and that also contains the double bond. If that chain has several double bonds, they are named looking for the smallest possible combination of the positions of those double bonds.
The position of the double bond It is indicated by inserting before the suffix -no the Latin prefix that indicates the number of the carbon where the double bond begins (di (2), tri (3), tetra (4), penta (5), octa (8), etc. ). The substituents (usually chlorine, bromine, ethyl, methyl, etc.) are named as prefixes (at the beginning of the name), detailed and in alphabetical order, in addition they are named looking for the smallest possible combination of their positions in the chain. For instance: 1-butene / 1,2-butadiene / 5-chloro-1-pentene / 4-pentenyl chloride
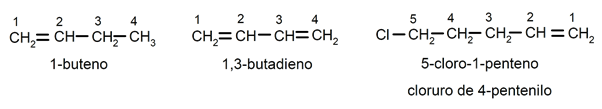
Given how complex the chemical name established according to the IUPAC criteriaMany naturally occurring organic alkenes have fancy names, often related to their natural source. For instance: limonene / 1-methyl-4- (1-methylethenyl) -cyclohexene / geraniol / 3,7-dimethyl-2,6-octadien-1-ol
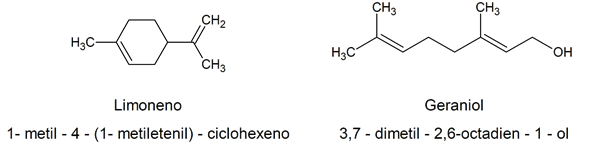
Alkenes of up to four carbons are gases at room temperature, those with 4 to 18 carbons they are liquid and the longest ones are solid. They are solubilized in organic solvents such as ether or alcohol and are slightly more dense than the corresponding alkanes (that is, with the same number of carbon atoms).
The melting and boiling point of alkenes, as in alkanes, increases the longer the carbon chain is.
On the other hand, due to the voltage generated by the double bond, the distance between the carbon atoms involved in the double bond in the alkene is 1.34 pm (picometers), while the distance of the single bond in the corresponding alkane is 1.54 pm.
They present a chemical reactivity much higher than alkanes, precisely because they have those double bonds that have a high electron density and that can be broken and allow the addition of other atoms, often hydrogen or halogens. They can also undergo oxidation and polymerization.
Alkenes usually present cis-trans isomerism or stereoisomerism, since the carbon atoms connected by the double bond cannot rotate and this causes the substituents to be located on the same side of the double bond or on opposite sides. For instance: trans-2-butene / cis-2-butene
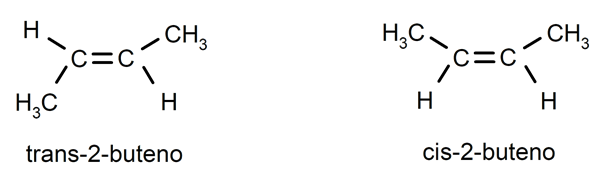
Alkenes with two double bonds are called dienes, and those with more than two double bonds are generally called polyenes.
In the plant world, alkenes are quite abundant and have physiological roles very significant, such as the regulation of the fruit ripening process or the filtration of certain solar radiation.
The chemical structure of organic alkenes is usually quite complex and includes carbon chains and rings. Some fruits (such as carrots or tomatoes) and some crustaceans (such as crabs) produce significant amounts of beta carotene, an important alkene that is a precursor of vitamin A.
Examples of alkenes
- ethene
- 2-methylpropene
- propene
- 2,3-butadiene
- myrcene
- limonene
- geraniol
- lycopene
- lanosterol
- mentofuran
- tetrafluoroethylene
- 1,3,5,7-cyclooctatetraene
- 5-bromo-3-methyl-3-hexene
- 3,5-diethyl-4-propyl-2,5-dimethyl-2-heptene
- 7,7,8-trimethyl-3,5-nonadiene
- 3,3-diethyl-1,4-hexadiene
- cyclohexene
- 1-chloro-2-methylprop-1-ene
- 3-bromo-5-methyl-cyclopentene
- 1-methyl-cyclohexene
Diagrams of the chemical compounds of these alkenes:


