The acids They make up an important and wide group of chemical compounds. Acids are generally defined as compounds that can donate one or more hydrogen cations (H+) to another compound, known as a base. However, acids are also defined according to various theories:
- Arrhenius theory. According to this theory, an acid is defined as a substance that can give off H+ ions when in solution. For example: HCl(aq) and HBr(aq).
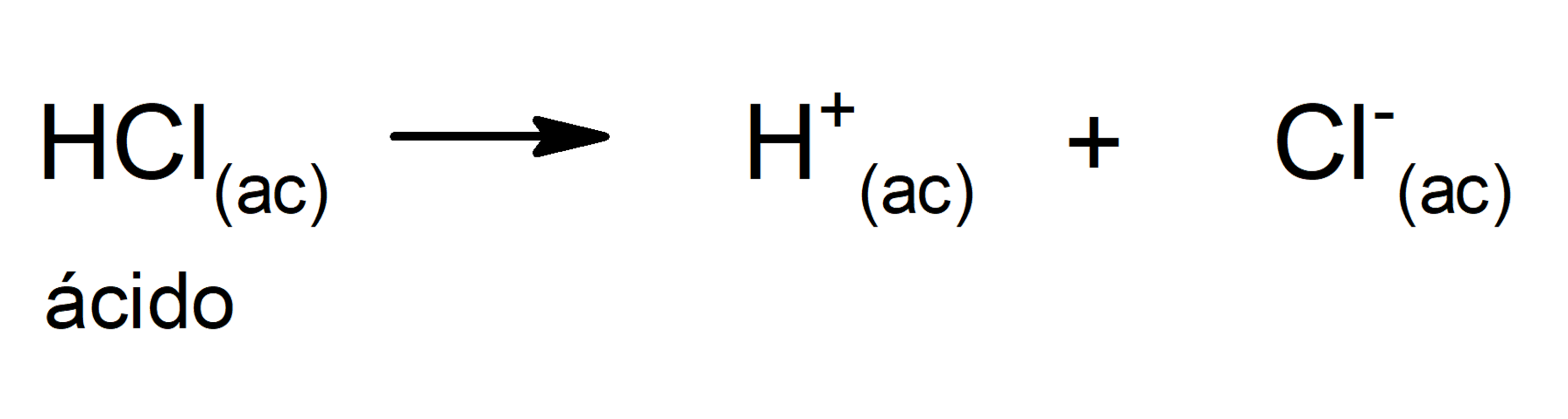 The limitation of this theory is that acids are defined only in aqueous solution.
The limitation of this theory is that acids are defined only in aqueous solution.
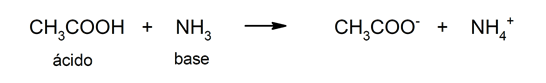
- Bronsted and Lowry theory. According to this theory, an acid is also defined as a substance that gives up an H+ cation, but gives it up to a base that accepts it. In this theory acids do not have to be defined in aqueous solution and, in addition, organic acids can also be explained. For example: acetic acid (CH3COOH).
- Lewis theory. According to this theory, an acid is any chemical species, molecule, or ion that accepts an electron pair from another substance. For example: the cation H3O+ (H+).

Properties of acids
Some of the main characteristics of acids are:
- They can be presented as liquids or gases, more rarely as solids.
- release hydrogen cations and it is what makes them produce solutions with a pH lower than 7. Acids that can release more than one proton (using this name for H+) are called polyprotic or polyfunctional.
- They are recognized by the acid taste that characterizes them, for example, in citrus fruits, which are rich in citric acid, or in vinegar, which is a solution of acetic acid (both organic acids).
- exist organic and inorganic acids, the strongest are usually inorganic. Many organic acids serve important biological functions, such as nucleic acids. Within the inorganic there is one, hydrochloric acid, which plays an important role in the digestion process.
- They are highly corrosive.
- Its strength is determined by the tendency to lose protons.
types of acids
Acids can be:
- strong acids. They are compounds with a great tendency to dissociate, so that nothing (or almost nothing) of the protonated acid remains in the solution. Strong acids are usually corrosive, to the point that they can cause skin burns. They are generally very good conductors of electricity. For example:
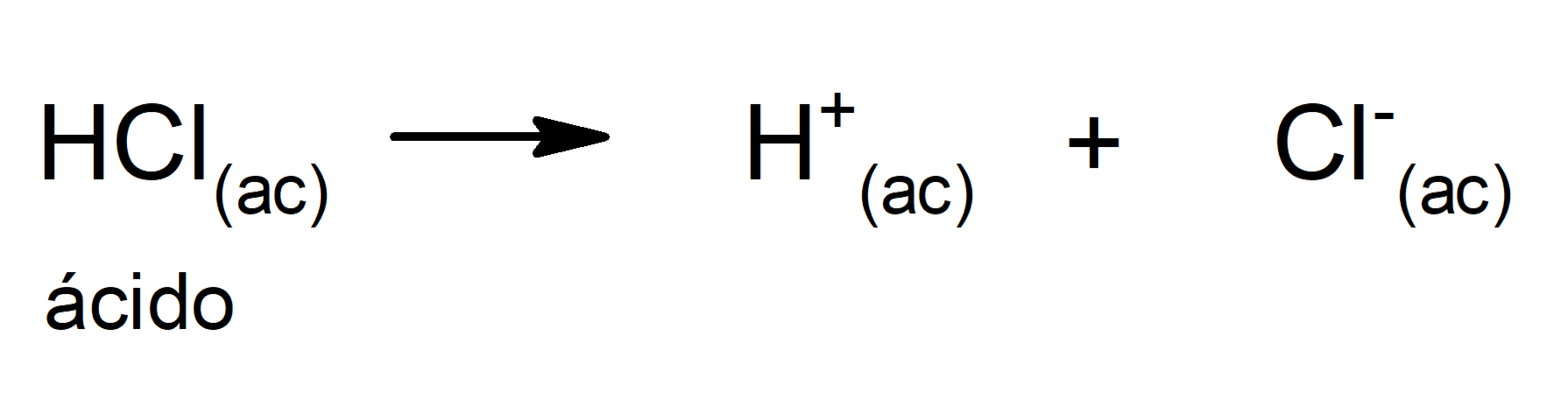
- weak acids. They are compounds that only partially dissociate, so that there is a balance between the dissociated and the undissociated form. For example:
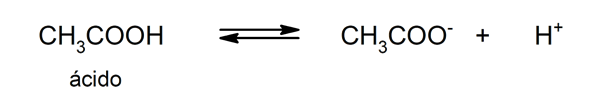
Examples of acids and their uses in everyday life
The acids have many usesboth on an industrial scale and at home. They are often used as additives and preservatives in food, cosmetics and beverages, as disinfectants and as catalysts (accelerator of chemical reactions) in the petrochemical or paper industry.
The main acids are:
- Perchloric acid (HClO4). It is a strong and liquid acid at room temperature, highly oxidizing and often used in industrial activity. It is used in the manufacture of electrical and electronic equipment, in batteries, in the refining of metals and in the manufacture of fertilizers and ammunition. In addition, in the home it is used as a cleaner and rust remover. This acid is also involved in the production of ammonium perchlorate, which is used for rocket propulsion, explosives, and fireworks.
- Nitric acid (HNO3). It is a strong and intensely oxidizing acid, used to make certain explosives, nitrogenous fertilizers, and as a laboratory reagent (it dissolves almost all metals).
- Ascorbic acid (C6H8OR6). It is also called vitamin C and is a necessary nutrient for human health. Ascorbic acid is a protective substance due to its antioxidant effects that supports the immune system, fights infections, protects tissues, heals wounds, among other functions. It is usually found in most fruits and vegetables.
- Hydrochloric acid (HCl(ac)). It is the only strong acid synthesized by the human body, a process that occurs in the stomach during the degradation of food in the digestive process. In addition, it is used industrially in food processing, in the production of steel and batteries. Hydrochloric acid is an ingredient in cleaners, disinfectants, and chemicals used in pool cleaning.
- Tartaric acid (C4H6OR6). It is a white crystalline powder that is used in the preparation of effervescent beverages, in the bakery, wine and pharmaceutical industries. The cream of tartar in some recipes is tartaric acid.
- Hydrofluoric acid (HF(ac)). It is a highly corrosive inorganic acid. Because of its ability to attack glass, it is used in crystal carving and engraving. In addition, it is used to obtain pharmaceutical compounds, as a cleaner and to obtain fluorinated organic compounds.
- Sulfuric acid (HtwoSW4). It is a strong acid that has many applications in various industries and synthesis processes, among which are: its use in the production of fertilizers, in the oil industry, in the production of batteries, among others.
- Trifluoroacetic acid (CtwoHF3ORtwo). It is a good solvent for many organic compounds, so it is mainly used in organic synthesis.
- phosphoric acid (H3PO4). It is an acid that is present in various cola drinks, in fertilizers, detergents, soaps and balanced food.
- Acetic acid (CH3COOH). It is the main component of vinegar and due to its acidity it is a widely used food preservative. This acid is also used in the manufacture of vinyl acetate, as a solvent and as a component of cleaners. It is also used in the process of photographic development and as a contrast material in the field of medicine.
- Fluoroantimonic acid (SbHF6). It is the strongest superacid known and is used to protonate (attach protons to an atom or molecule) organic compounds.
- Chromic acid (HtwoCrO4). It is a dark red powder that participates in the chrome plating process. In addition, it is used to glaze ceramics, to color and clean glass, in the manufacture of paints, and in the leather and wood industries.
- Indoleacetic acid (C10H9NOtwo). It is the main representative of auxins, phytohormones that intervene in the process of plant growth. This process includes germination, root and fruit growth, leaf growth, among others.
- Deoxyribonucleic acid (DNA). It is a nucleic acid that contains all the genetic information of the organism. DNA makes up the genes that govern the synthesis of countless proteins.
- tricarboxylic acids. It is a group of carboxylic acids that has three carboxyl groups (-COOH). Citric acid (C6H8OR7) is a type of tricarboxylic acid found in many fruits and operates in the body as an antioxidant.
- Formic acid (CHtwoORtwo). It is the simplest of organic acids and is used in the leather industry, in the chemical industry, to preserve feed for livestock, in the poultry industry and in textiles.
- Gluconic acid (C6H12OR7). It is an acid that has salts that are used in cleaning processes for glass objects.
- lactic acid (C3H6OR3). It is an acid that is used as an additive in the food industry and to control acidity. It is also used in the cosmetic industry, in anti-aging products.
- Benzoic acid (C7H6ORtwo). It is an acid with a characteristic odor that is used to preserve foods that require an acidic pH. It is also used as a component in toothpaste, in perfumery, in the tobacco industry, and to soften plastics.
- Malic acid (C4H6OR5). It is an organic acid widely used in the pharmaceutical industry to produce laxatives. In addition, it is used in the food industry, as a flavoring or preservative, and in the wine industry.
- Carbonic acid (HtwoCO3). It is an acid that comes from carbon dioxide and is present in foods and beverages, such as soft drinks, beers and some dairy products. Also, it is found in dry ice and effervescent tablets. Carbonic acid is used in the chemical industry, in refrigeration chambers and in fire-extinguishing systems.
