In inorganic chemistry salts are called compounds that are obtained when an acid is replaced by its hydrogen atoms by metallic cations (although sometimes by non-metallic cations, for example, the ammonium cation, NH4+). In the specific case of acid salts, the hydrogens of the acid are partially substituted, that is, at least one unsubstituted hydrogen remains as part of the salt. In this they differ from neutral salts, where the hydrogens of the acid are totally replaced.
Salts are usually formed through the reaction between an acid and a hydroxide (base). In these reactions, normally the base loses its hydroxyl groups (-OH) and the acid the hydrogen atoms (H), forming a neutral salt; but if the acid in question conserves at least one of its hydrogen atoms, altering the electric charge of the reaction, we will obtain an acid salt or a hydrogenated salt.
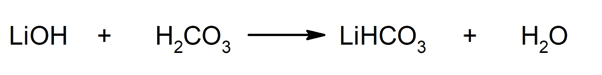
Thus, for example, lithium bicarbonate and water are obtained from the reaction between lithium hydroxide and carbonic acid:
Nomenclature of acid salts
Acid salts can be named according to three types most common nomenclature:
- Traditional nomenclature. The salt is named as if it were neutral, but the word ‘acid’ is placed before the name of the metal or the prefix (bi-) before the name of the salt. For example: lithium acid carbonate or lithium bicarbonate (LiHCO3).
- Stock nomenclature. The salt is named as if it were neutral but with the word ‘hydrogen’ before its name. For example: lithium hydrogen carbonate (LiHCO3).
- Systematic nomenclature. The salt is named by putting the word ‘hydrogen’ followed by the prefix that indicates the amount of oxygen (di-, tri-, tetra-), then the word ‘oxo’ is put followed by the name of the salt anion as if it were neutral Indicating in Roman numerals and in parentheses the oxidation number of the nonmetal that makes up its anion, finally it is put ‘from the name of the metal’. For example: Lithium hydrogen trioxocarbonate (IV) (LiHCO3).
Examples of acid salts
- Sodium bicarbonate (NaHCO3). Also called sodium hydrogen carbonate (IV), it is a white crystalline solid, soluble in water, which can be found in nature in a mineral state or can be produced in the laboratory. It is one of the best known acid salts and is widely used in confectionery, pharmacology or yogurt making.
- Lithium bicarbonate (LiHCO3). This acidic salt has been used as a capturing agent for COtwo in situations where such gas is undesirable, as in the North American “Apollo” space missions.
- Potassium dihydrogen phosphate (KHtwoPO4). It is a crystalline solid, odorless, soluble in water, widely used in various industries such as food yeast, chelating agent, nutritional fortifier and assistant in fermentation processes.
- Sodium bisulfate (NaHSO4). It is an acid salt formed by the neutralization of sulfuric acid. It is widely used industrially in the refining of metals and cleaning products. Although it is highly toxic to some echinoderms, it is used as an additive in pet food and in the manufacture of jewelry.
- Sodium hydrogen sulfide (NaHS). It is a dangerous compound to handle, since it is highly corrosive and toxic. It can cause severe skin burns and eye damage as it is also combustible.
- Calcium hydrogen phosphate (CaHPO4). It is used as a dietary supplement in cereals for cattle. It is a solid insoluble in water but capable of crystallizing when hydrated, consuming two molecules of water.
- Ammonium hydrogen carbonate ([NH4]HCO3). Also known as “ammonium bicarbonate”, it is used in the food industry as chemical yeast, although it has the disadvantage of trapping ammonia and giving food a bad taste if used in excess. It is also used in fire extinguishers, pigment making, and to expand rubber.
- Bari bicarbonateor (Ba[HCO3]two). It is an acid salt that, when heated, can reverse its production reaction and is highly unstable, except in solution. It is widely used in the ceramic industry.
- Sodium bisulfite (NaHSO3). This salt is extremely unstable and in the presence of oxygen it derives into sodium sulfate (NatwoSW4), which is why it is used in the food industry as a food preservative and desiccant. It is an extreme reducing agent and commonly used by man, also used in fixing colors.
- Calcium citrate (AC3[C6H5O7]two). Commonly known as bitter salt, it is used as a food preservative and as a nutritional supplement when it is linked to the amino acid lysine. It is a white, odorless, crystalline powder.
- Monocalcium phosphate (AC[H2PO4]two). It is a colorless solid that is obtained from the reaction of calcium hydroxide and phosphoric acid, widely used as a leavening agent or as a fertilizer in agricultural work.
- Dicalcium phosphate (CaHPO4). Also known as calcium monohydrogen phosphate, it has three different crystalline forms that are used as an additive in food and is present in toothpastes. In addition, it is naturally formed in kidney stones and the so-called “kidney stone”.
- Monomagnesium phosphate (MgH4PtwoOR8). It is a white, crystalline and odorless salt, partially soluble in water. It is used in the preservation of food, as an acidulant, as an acidity corrector or an agent in the treatment of flours.
- Sodium diacetate (NaH[C2H3O2]two). This salt is used as a flavoring and preservative agent for meals, which prevents or delays the appearance of fungi and mycobacteria, both in vacuum packed products such as meat products and in the flour industry.
- Calcium bicarbonate (AC[HCO3]two). It is a hydrogenated salt that originates from calcium carbonate, present in minerals such as limestone, marble and others. This reaction involves the presence of water and COtwo, so it can occur spontaneously in caves and caves rich in calcium.
- Rubidium acid fluoride (RbHF). This salt is obtained from the reaction of hydrofluoric acid (hydrogen and fluorine) and rubidium, an alkali metal. The result is a toxic and corrosive compound that must be handled with caution.
- Monoammonium phosphate ([NH4]HtwoPO4). It is a water soluble salt produced by the reaction of ammonia and phosphoric acid. It is usually used as a fertilizer since it provides the soil with the nitrogen and phosphorus nutrients necessary for plant growth. It is also part of the ABC powder in fire extinguishers.
- Zinc hydrogen orthoborate (Zn[HBO3]). It is a salt used as an antiseptic and as an additive in the manufacture of ceramics.
- Monosodium phosphate (NaHtwoPO4). It is used mostly in laboratories, as a “buffer” or buffer solution, which prevents sudden changes in the pH of a solution.
- Potassium hydrogen phthalate (KHP). Also called “potassium acid phthalate”, it is a solid and stable salt in ordinary air, which is why it is often used as a primary standard in pH measurements. It is also useful as a buffer agent in chemical reactions.
