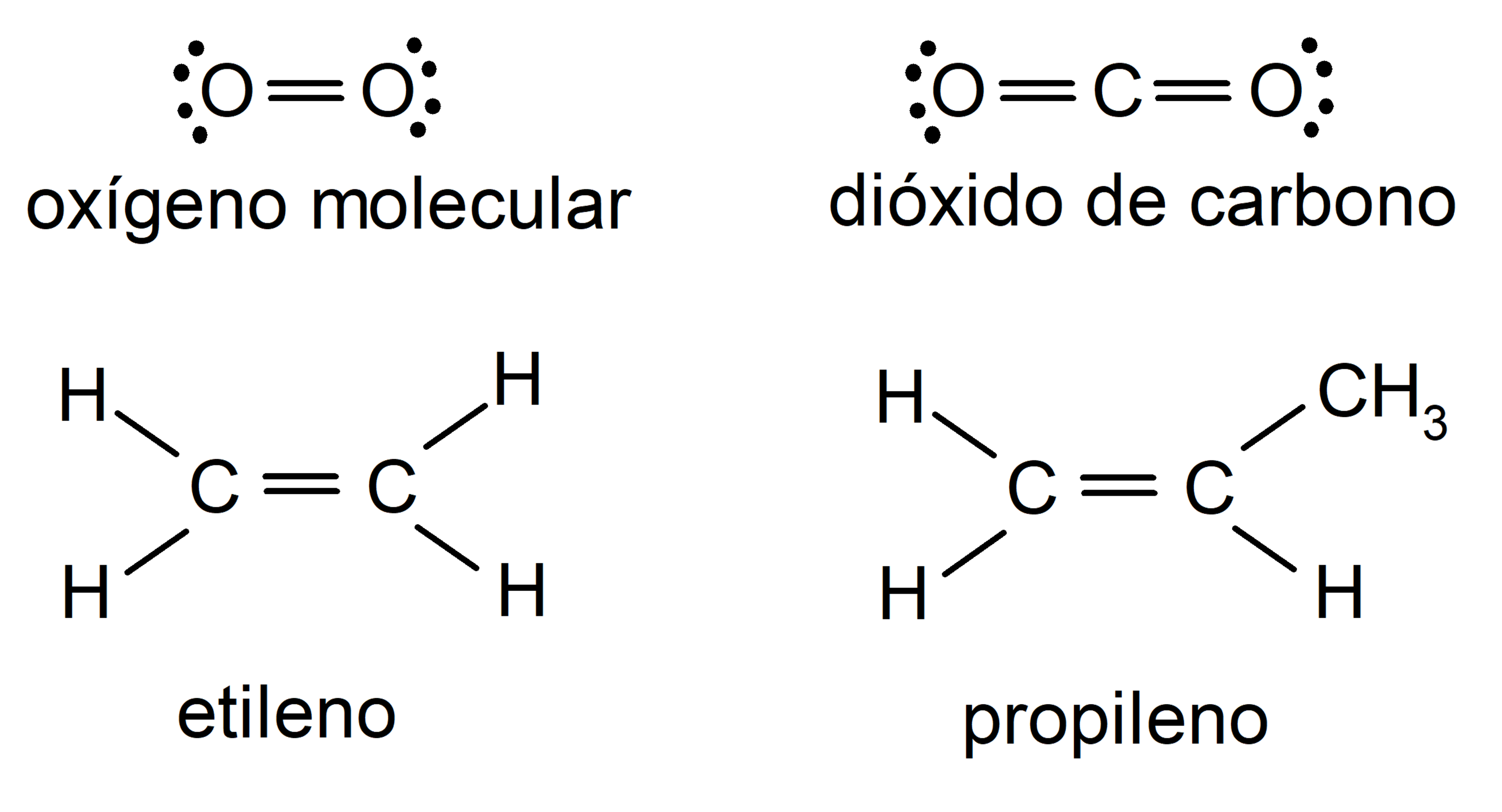In chemistry, when we talk about single, double and triple bonds we are referring to three of the known forms of covalent bonding, that is, bonds between atoms from the sharing of electrons from their last energy levels. There is also the dative covalent bond, in which case the electrons that are shared are contributed by only one of the atoms that form the bond.
Covalent bonds occur when two atoms are brought close enough together to partially overlap their bonds. atomic orbitals (region of space where an electron is likely to be found around the nucleus), thus compensating for the simultaneous attractive and repulsive forces arising from their electrical charges (negative electrons repel each other but the positive charges of atomic nuclei repel them). attract) and acquiring a maximum stability that allows them to constitute a molecule.
When this happens, the electron pairs that orbit the outermost shells of each atomic nucleus are displaced towards each other until it is impossible to determine to which atomic nucleus they belonged.
Differences between covalent bond, ionic bond and metallic bond
- covalent bonds
Covalent bonds form between atoms of non metallic elements, either of the same element or of different elements. They also form between a nonmetal and hydrogen. For such a bond to form, the electronegativity difference between the bonding atoms must be less than 1.7.
If the atoms that make up a covalent bond are of different nonmetals, one of them will have greater electronegativity than the other. For this reason, the electrons of the bond will be more attracted by the more electronegative atom, leaving a negative charge density on it, while a positive charge density will remain on the less electronegative atom, which generates a dipole (system of two electric charges of opposite sign that are very close) molecular. In these cases, a polar covalent bond, which constitutes the polar molecules that can attract other molecules of the same compound.
On the other hand, if the atoms involved in the covalent bond are of the same nonmetallic element, they will not be created. dipoles, so the electronegativity difference between them will be zero and there will be no attraction between molecules of the same compound. The bond of this type of molecules is called “apolar covalent bond”.
- ionic bonds
The ionic bonds They form between atoms with a high electronegativity difference. A transfer of at least one electron occurs from the less electronegative atom (forming a (+) cation) to the more electronegative atom (forming a (-) anion). The bond is finally formed by electrostatic attraction between both ions of opposite charges.
- metallic bonds
The metal links are formed between atoms of metallic elements. The atoms of these types of elements are very close together, and the valence electrons form a “cloud” around them.
Types of covalent bond
Depending on the number of shared electron pairs between the atoms that form the bond, covalent bonds will be single (one pair), double (two pairs), or triple (three pairs). Each is usually represented by one, two or three lines between the symbols of each atom:
- H – H, the H moleculetwo via a single link.
- O = O, the O moleculetwo through a double bond.
- N Ξ N, the N moleculetwo through a triple bond.
The number of single, double or triple bonds that an atom can form depends directly on its valence, that is, on the number of electrons it can share from its outermost shell.
In addition, depending on the degree of complexity of the bond, the molecule enjoys more or less mobility (less mobility the more complex the bond) since the distance between the atoms is smaller and it is more difficult to break the bond, that is, it is necessary to apply more energy to achieve your break.
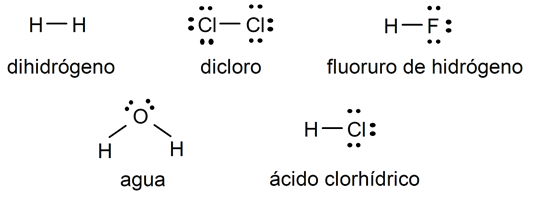
examples of simple covalent bond
- Hydrogen molecule (Htwo)
- Chlorine molecule (Cltwo)
- Hydrogen fluoride (HF) molecule
- water molecule (HtwoOR)
- Hydrochloric acid (HCl) molecule

examples of double covalent bond
- Oxygen molecule (Otwo)
- Carbon dioxide molecule (COtwo)
- Ethylene molecule (CtwoH4)
- Propylene molecule (C3H6)

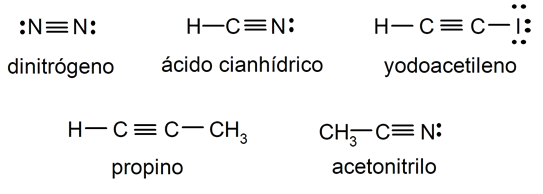
examples of triple covalent bond
- Nitrogen molecule (Ntwo)
- Hydrocyanic acid (HCN) molecule
- Molecule of iodoacetylene (HCtwoI)
- Propyne molecule (C3H4)
- Acetonitrile molecule (CH3NC)