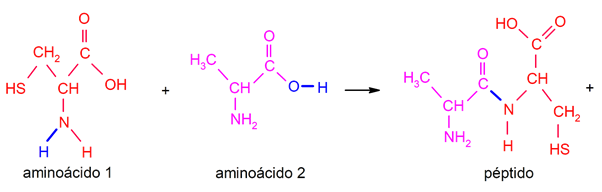
The peptide bonds are a specific type of bond between one amino acid and another, which takes place through an amino group (-NHtwo) in the first amino acid and a carboxyl group (-COOH) in the second, producing a covalent bond -CO-NH- and releasing a water molecule. For example: oxytocin, vasopressin, leptin.
In this way a new molecule called peptide and that will bear the name of both amino acids. The peptide bond between a molecule of to the girl (providing the terminal -NHtwo) and another of serine (providing the -COOH terminus) is named as an alanyl-serine peptide.
This is one of the bond forms that allow joining amino acids (by dehydration) to produce more complex structures (polypeptides) since once the link is obtained, it is possible to continue joining amino acids through the same process, starting from the terminal carboxyl group. In this same way, more complex structures such as polypeptides and proteins can be obtained. It is an extremely common procedure in living beings.
properties of peptide bonds
Peptide bonds have certain characteristics. For example, the bond established is of a simple but shorter type: with characteristics of a double bond, such as being stabilized by resonance. The latter prevents free turns around the bond (something common in this type of union), which gives the peptide bond an unavoidable planar structure.
Similarly, peptide bonds can be degraded or broken by hydrolysis (addition of water), releasing a quantity of energy in a very slow process. This process can be accelerated in the presence of acidic, basic or enzymatic catalysts.
examples of peptide bonds
Any peptide is a perfect example of peptide bonds, as they are the result of this type of joining of amino acids. Here are some of the most important:
- bradykinin (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg). Composed of nine amino acids, this peptide is a drug that produces vasodilation and a drop in blood pressure, which is why it is used to treat hypertensive patients.
- oxytocin (Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly). It is a hormone produced by the hypothalamus that performs neuromodulatory functions of the central nervous system and plays a vital role in preparing the female cervix during childbirth and the breasts during lactation.
- glucagon (NH2-His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln -Trp-Leu-Met-Asn-Thr-COOH). It is a peptide hormone of 29 amino acids that is synthesized in the pancreas and is involved in the metabolism of sugars.
- Glutathione (L-γ-glutamyl-L-cysteinylglycine). It is a tripeptide of three amino acids: cysteine, glutamate and glycine. It is the main cellular antioxidant, which protects cells from free radicals and peroxides.
- Vasopressin (NHtwo-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-COOH). Secreted by the hypothalamus, it controls the reabsorption of water molecules from urine, increases their concentration and plays a key role as a blood homeostatic regulator. It is a hormone of nine amino acids.
- Insulin. It is a polypeptide hormone made up of 51 amino acids, secreted by the pancreas to regulate the cycle of blood sugars.
- prolactin. It is a peptide hormone that stimulates the production of milk in the mother’s breasts. It is made up of a sequence of 198 amino acids.
- leptin. It is another peptide hormone that suppresses the feeling of hunger and is made up of a chain of 167 amino acids.
- Gastrin. It is a peptide hormone that regulates the production of gastric juices in the stomach. It is made up of 14 amino acids.
- Pepsin. It is a hormone composed of 326 amino acids, responsible for regulating the processes of digestion and absorption of food.
