[ad_1]
Matter is everything that has mass and body and occupies a place in space. It can be found in three states: liquid, solid, and gaseous (although today, we know of a fourth state of aggregation, plasma). Each state has physical characteristics that characterize it.
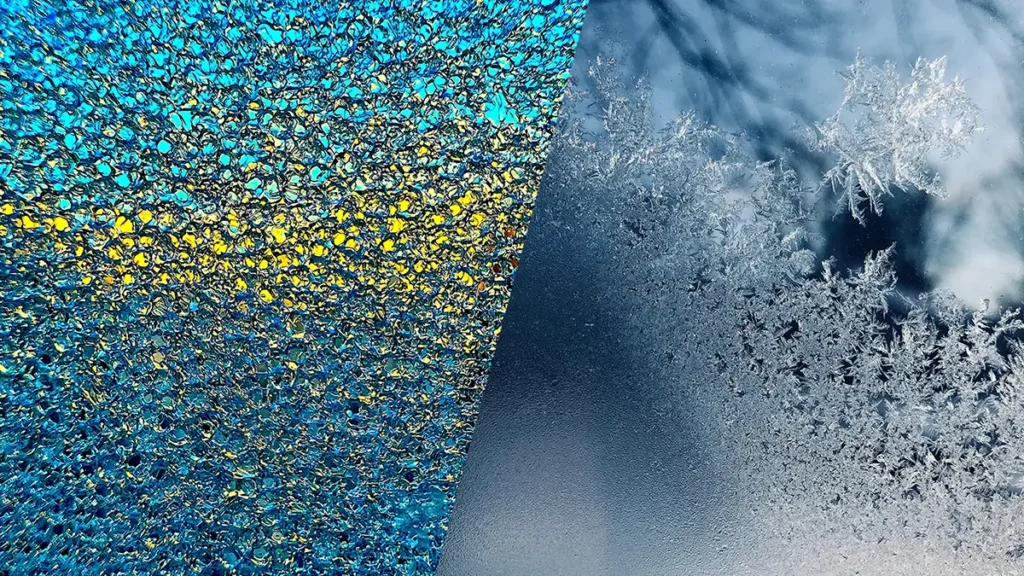
When matter is exposed to large changes in pressure and/or temperature, it can experience a change in status (from solid to gas, from liquid to solid, from gas to liquid, and vice versa). In all cases where a change in the state of matter occurs, it is not transformed into another substance but changes its physical appearance without altering its chemical composition.
The phenomena that occur when matter goes from a solid state (has a defined shape) to a gaseous state (has no defined volume or shape and expands freely) and vice versa are:
- Sublimation. The phenomenon by which matter passes from the solid to the gaseous state without passing through the liquid state. For example, naphthalene tablets that gradually degrade from solid to gas, dry ice (dry carbon dioxide). The solid substance absorbs energy in the form of heat from its surroundings to pass into the gaseous state.
- Reverse deposition or sublimation. The phenomenon by which matter passes from the gaseous state to the solid state. Gaseous particles stick together more than usual and go directly to the solid state without going through the liquid state. This type of change is generally given by a decrease in temperature and under certain pressure conditions. For example, the formation of snow or frost. This process releases energy.
However, in most cases, the substance goes from a gaseous state to a liquid state (condensation or liquefaction) and from there to a solid state. The change from gaseous to solid state (and vice versa) occurs under specific pressure and temperature conditions.
Examples of solid to gaseous (sublimation)
- Solid sulfur. It sublimates at high temperatures and transforms into gases with a high level of toxicity.
- Solid iodine. After sublimation, it becomes a violet gas.
- Arsenic. At atmospheric pressure, it sublimates at 613 °C.
- Ice or snow. It can sublimate at temperatures below 0 °C.
- Benzoic acid. It sublimates above 390 °C.
- Camphor. Sublimes at a certain temperature.
- Flavoring tablet. Sublimates gradually like naphthalene.
Examples of gaseous to solid (reverse sublimation)
- Soot. In a hot and gaseous state, it rises, comes into contact with the chimney’s walls, and solidifies.
- Snow. Low temperatures cause water vapor in clouds to turn into snow.
- Iodine crystals. When heated, vapors are produced, which are transformed again into iodine crystals in contact with a cold object.
[ad_2]
